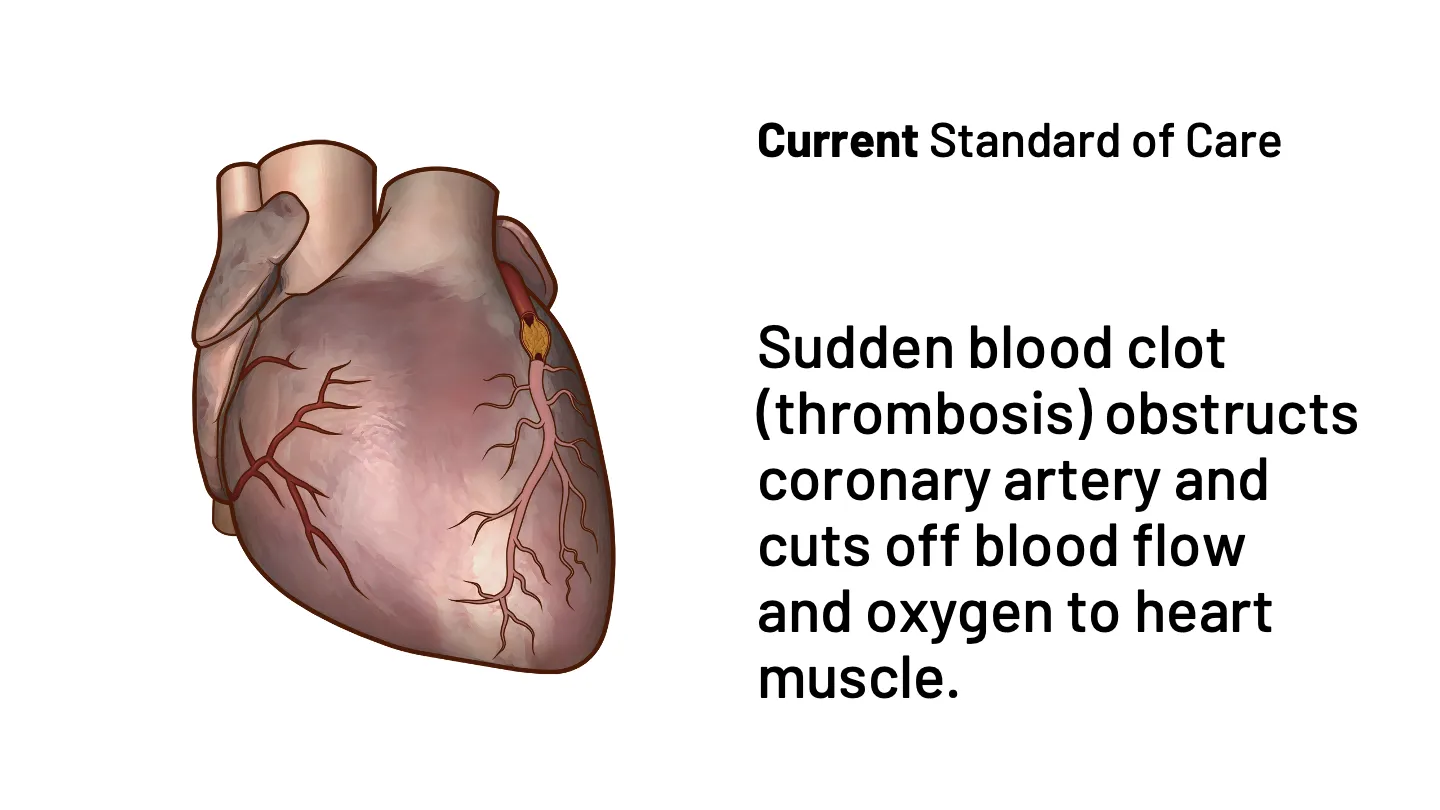
Sarah is having a heart attack. What happens in the next hour will determine if she survives – and the quality of the rest of her life.
Breakthrough Therapy:
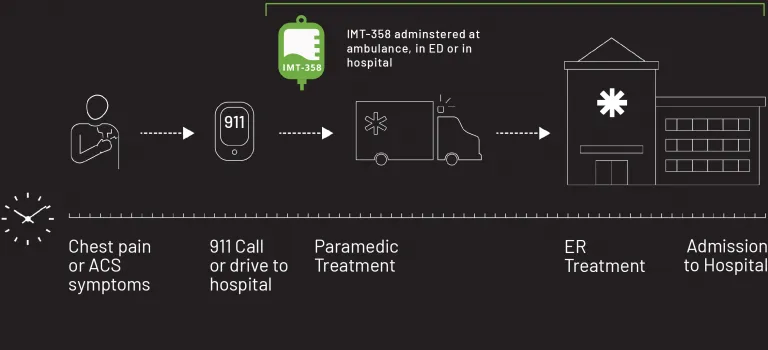
If Sarah had the option to receive earlier intervention in the ambulance following a 911 call, or in the ER, there could be an opportunity to reduce the risks to her clinical prognosis. That is why IMMEDIATE is developing IMT-358. FDA has designated IMT-358 as a Breakthrough Therapy for the treatment of acute coronary syndrome to reduce the risk of mortality, hospitalization for heart failure, and cardiac arrest.
IMT-358:
IMT-358: An investigational drug under development by IMMEDIATE Therapeutics to be administered the first hours of a developing heart attack (i.e., ACS) to provide metabolic protection to the heart muscle, potentially minimizing cardiac damage and reducing cardiac instability that can lead to cardiac arrest. The efficacy of IMT-358 is under confirmation in a planned pivotal phase 3 trial.



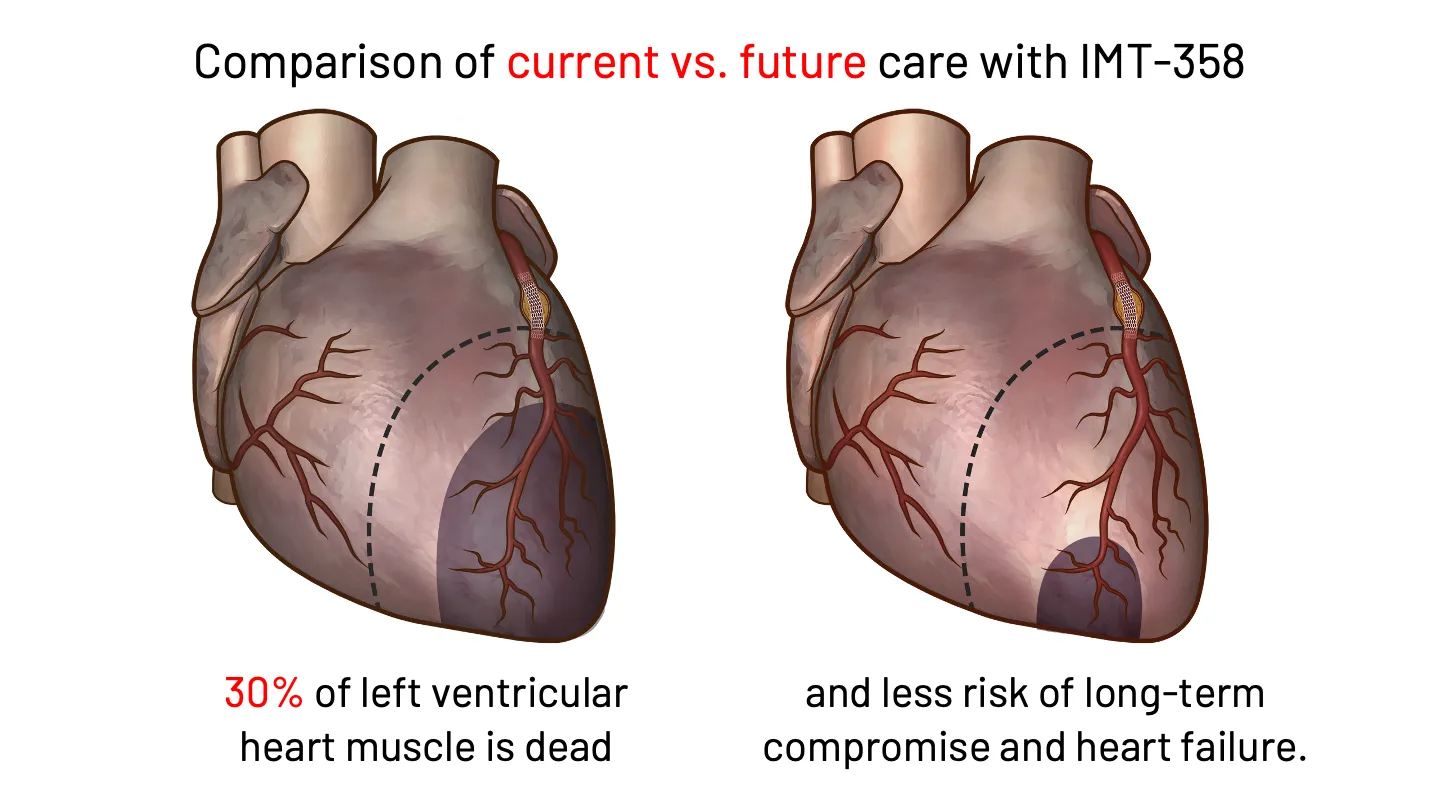
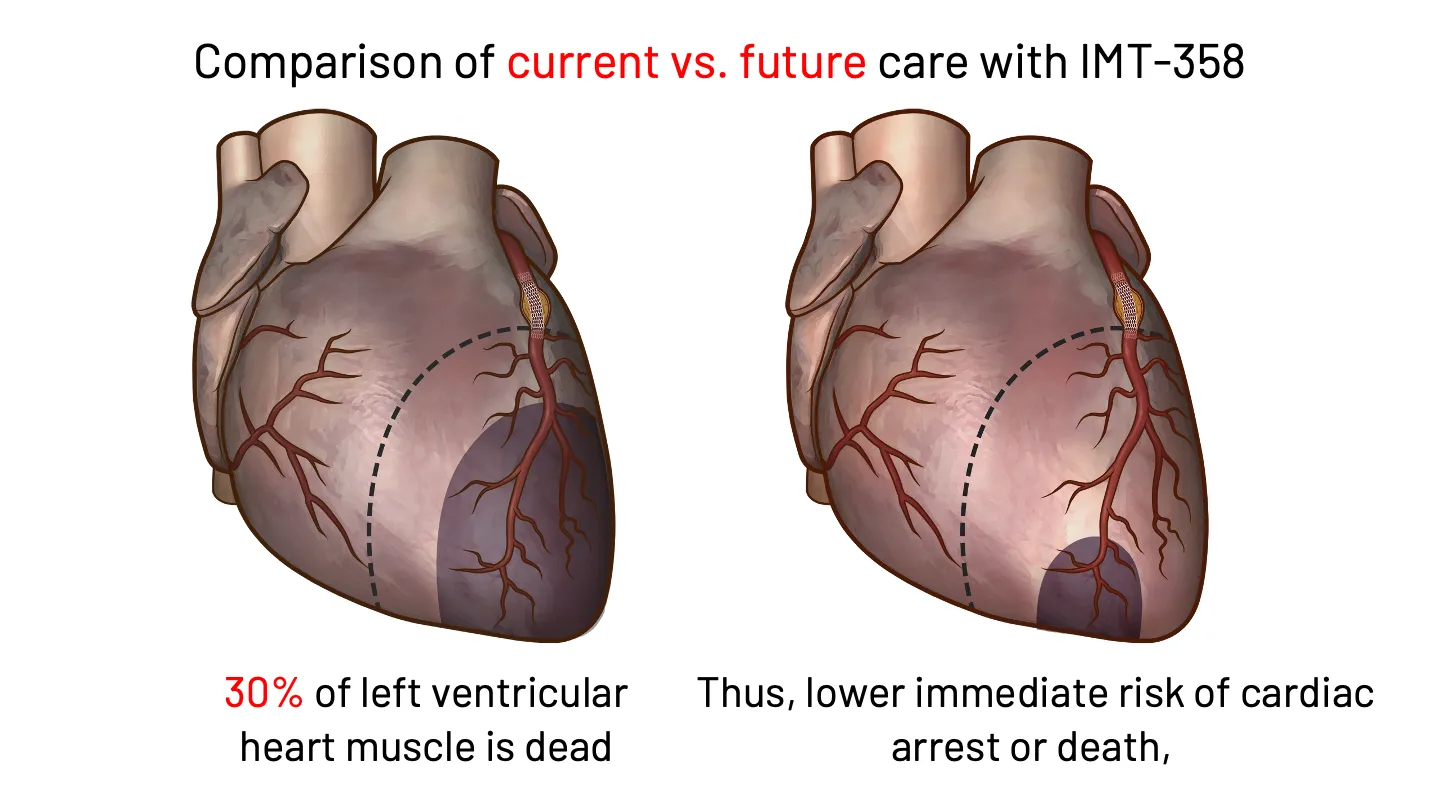
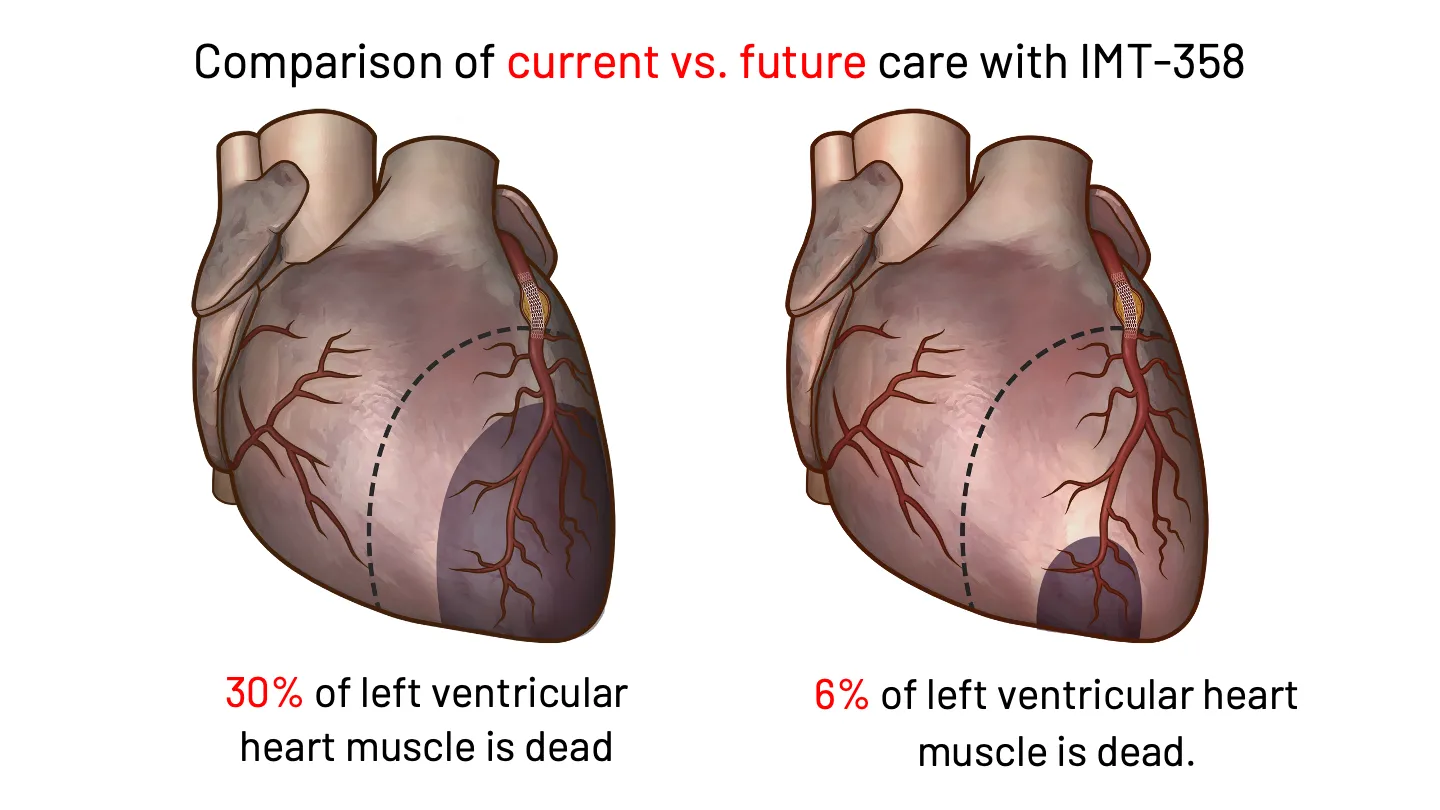
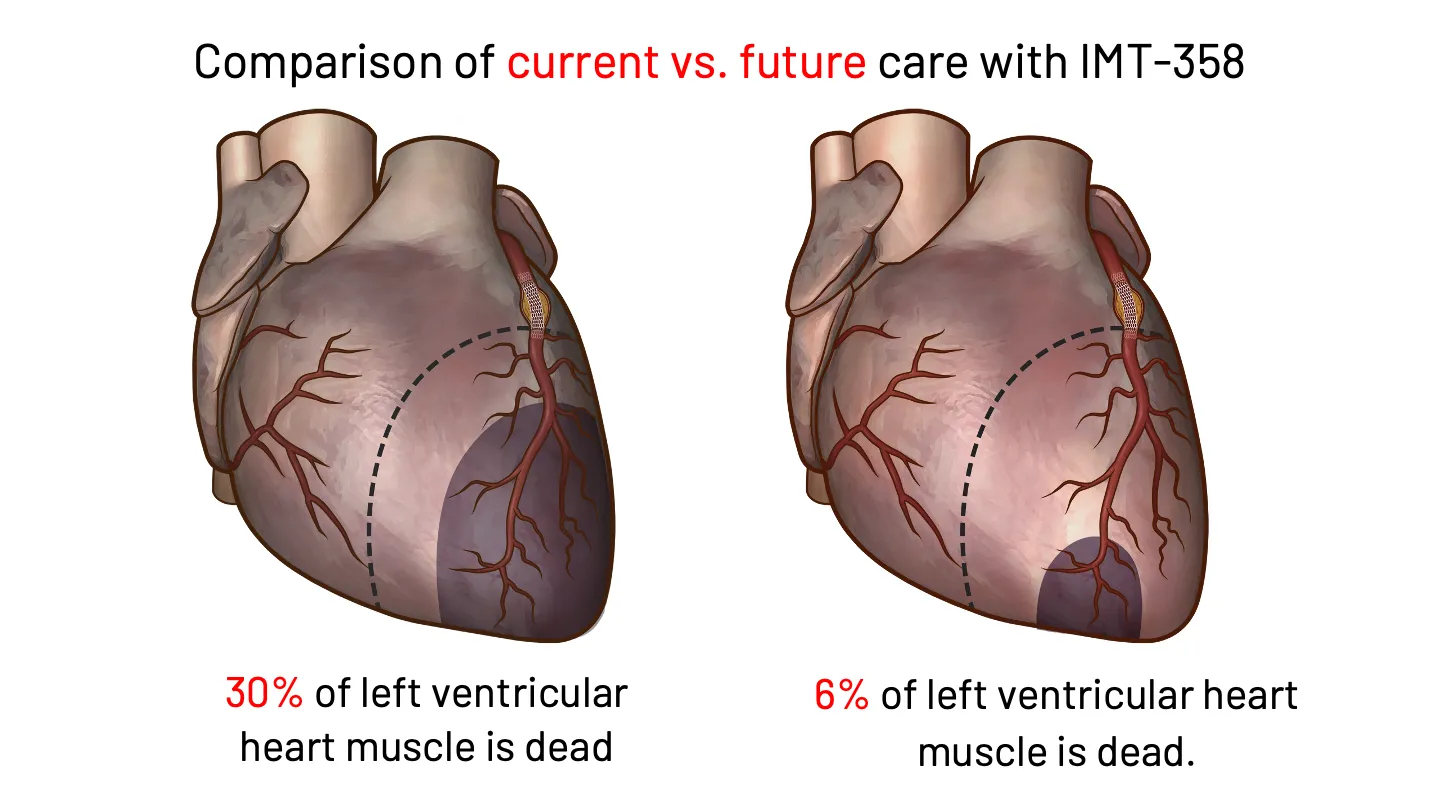
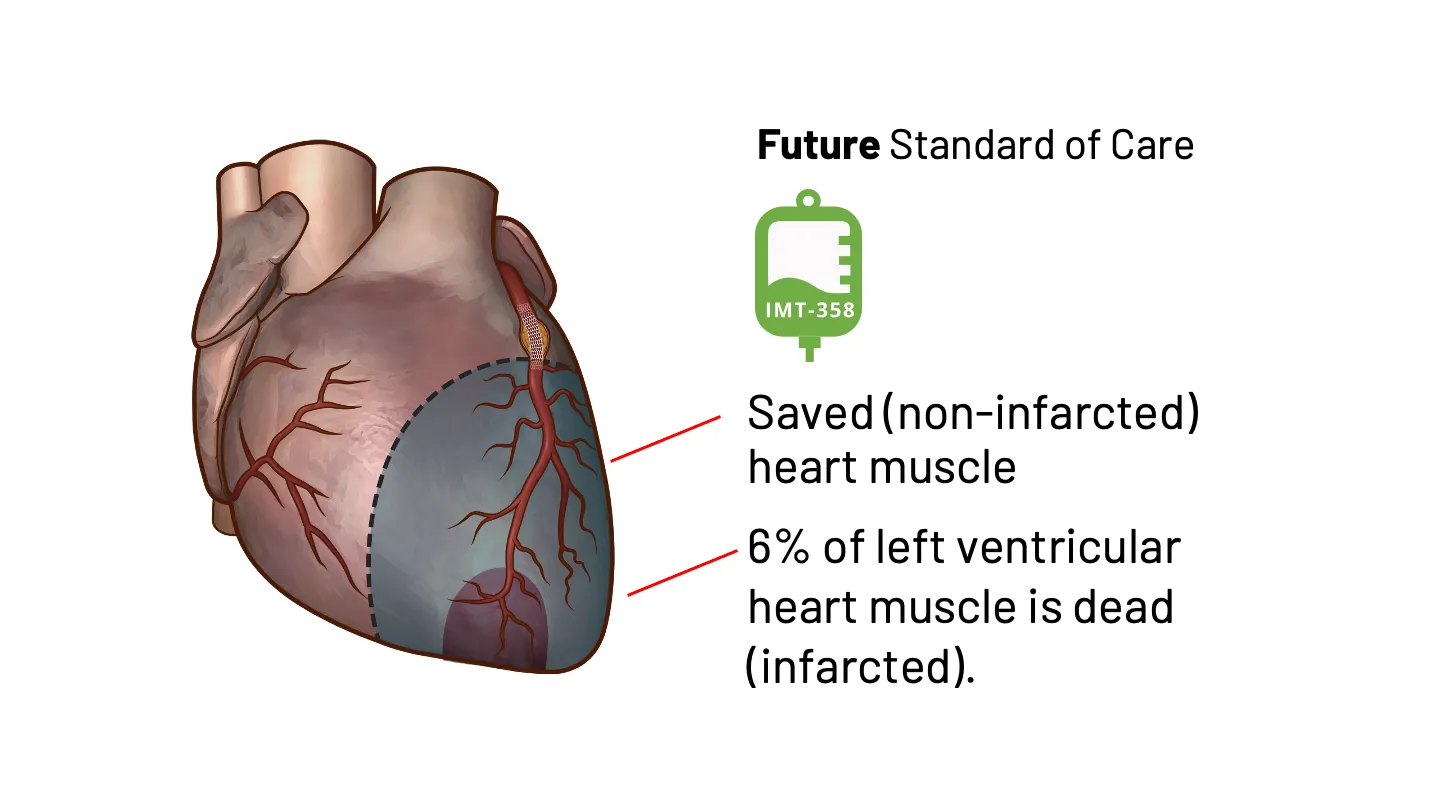




IMT-358 Begins When Time is Most Critical
Leadership Team
- Former Chief Strategy Officer, Peptilogics with recent experience in raising $78M in 2025
- Former Chief Strategy Officer & Head of Ops, Harbour BioMed with recent IPO experience raising $400M in 2020
- Former Global Ops. Lead, Dupixent Franchise, Sanofi
- Former Head, Asia Pacific R&D Strategy, Sanofi
- Several Years Consulting Experience across Pharma Value Chain
- Executive Director, Institute for Clinical Research and Health Policy Studies; Pratt Diagnostic Center Primary Care Physician
- Dean, Clinical and Translational Science Institute; Prof. of Medicine, Tufts University School of Medicine
- Chief, Division of Cardiology, Tufts Medical Center
- Professor of Medicine and Radiology, Tufts Univ School of Medicine
- Former Ad-hoc Member, FDA Cardiorenal AdCom
- Ad-hoc Member of the Central and Peripheral Nervous System AdCom and the Medical Imaging Drugs AdCom


